
Stimulated emission
Stimulated emission was a theoretical discovery by Einstein
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons (except in the case of hydrogen-1, which is the only stable nuclide with no neutrons). The electrons of an atom are bound to the nucleus by the electromagnetic force.
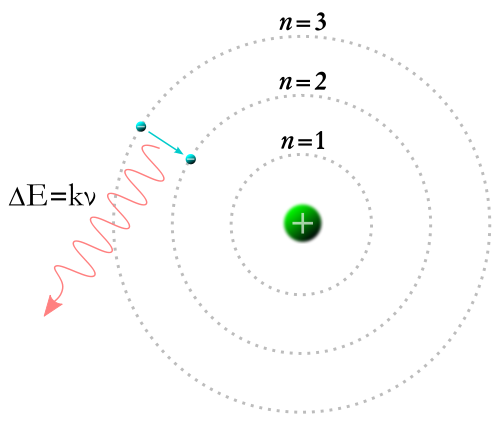
A Bohr model of the hydrogen atom, showing an electron jumping between fixed orbits and emitting a photon of energy with a specific frequency
When an electron is bound to an atom, it has a potential energy that is inversely proportional to its distance from the nucleus. This is measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). In the quantum mechanical model, a bound electron can only occupy a set of states centered on the nucleus, and each state corresponds to a specific energy level. The lowest energy state of a bound electron is called the ground state, while an electron at a higher energy level is in an excited state.
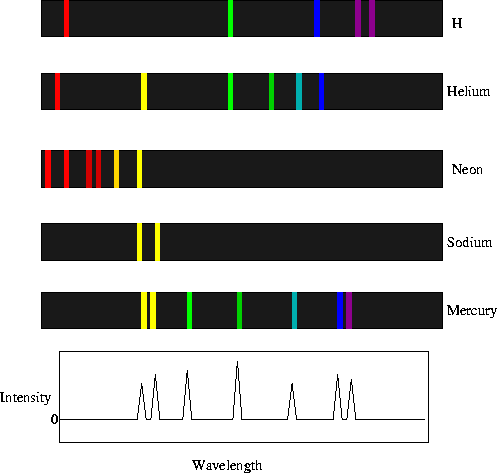
For an electron to transition between two different states, it must absorb or emit a photon at an energy matching the difference in the potential energy of those levels. The energy of an emitted photon is proportional to its frequency, so these specific energy levels appear as distinct bands in the electromagnetic spectrum. Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors
Spontaneous emission
Spontaneous emission is the process by which a light source such as an atom, molecule, nanocrystal or nucleus in an excited state undergoes a transition to a state with a lower energy, e.g., the ground state and emits a photon. Spontaneous emission of light or luminescence is a fundamental process that plays an essential role in many phenomena in nature and forms the basis of many applications, such as fluorescent tubes, older television screens (cathode ray tubes), plasma display panels, lasers (for startup - normal continuous operation works by stimulated emission instead) and light emitting diodes.
When an electron absorbs energy either from light (photons) or heat (phonons), it receives that incident quanta of energy.
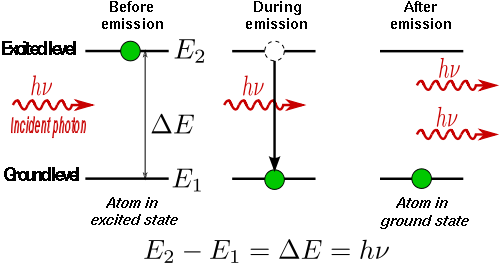
When an electron is excited from a lower to a higher energy level, it will not stay that way forever. An electron in an excited state may decay to a lower energy state which is not occupied, according to a particular time constant characterizing that transition. When such an electron decays without external influence, emitting a photon, that is called "spontaneous emission". The phase associated with the photon that is emitted is random. A material with many atoms in such an excited state may thus result in radiation which is very spectrally limited (centered around one wavelength of light), but the individual photons would have no common phase relationship and would emanate in random directions. This is the mechanism of fluorescence and thermal emission.
Stimulated emission
In optics, stimulated emission is the process by which an atomic electron (or an excited molecular state) interacting with an electromagnetic wave of a certain frequency may drop to a lower energy level, transferring its energy to that field. A photon created in this manner has the same phase, frequency, polarization, and direction of travel as the photons of the incident wave. This is in contrast to spontaneous emission which occurs without regard to the ambient electromagnetic field. However the process is identical in form to atomic absorption in which the energy of an absorbed photon causes an identical but opposite atomic transition: from the lower level to a higher energy level. In normal media at thermal equilibrium, absorption exceeds stimulated emission because there are more electrons in the lower energy states than in the higher energy states. However when a population inversion is present the rate of stimulated emission exceeds that of absorption, and a net optical amplification can be achieved. Such a gain medium, along with an optical resonator, is at the heart of a laser or maser. Lacking a feedback mechanism, laser amplifiers and superluminescent sources also function on the basis of stimulated emission
Laser